Dynamic Equilibrium Between Liquid and Vapor Is Established When
Any vessel containing water that is open to the atmosphere will try to establish that equilibrium but that wont be complete until the air is 100 saturated with vapour humidity100. At least 2 states of a system must be simultaneously present coexist.

11 5 Vaporization And Vapor Pressure Chemistry Libretexts
Chemistry questions and answers.

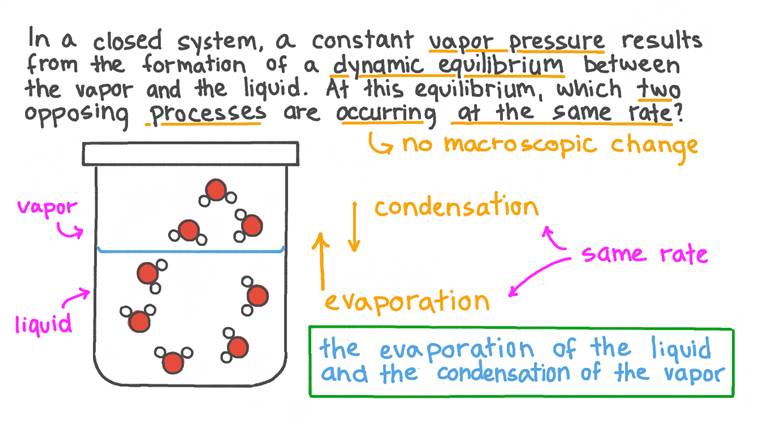
. In a closed container there is dynamic equilibrium between the vapour phase and the liquid phase and the rate of evaporation is equal to the rate of condensation. Because it is a continuous process until it is disturbed. The rate of evaporation is not zero so the vapor.
So the vapor pressure remains constant as there is an equilibrium between the liquid and vapor phase as the number of molecules leaving the liquid phase are equal to the number of molecules returning to the liquid phase. Br 2 l Br 2 g This computer animation shows the vapor pressure of bromine above liquid bromine at the. In other words the rate of evaporation is equal to the rate of condensation.
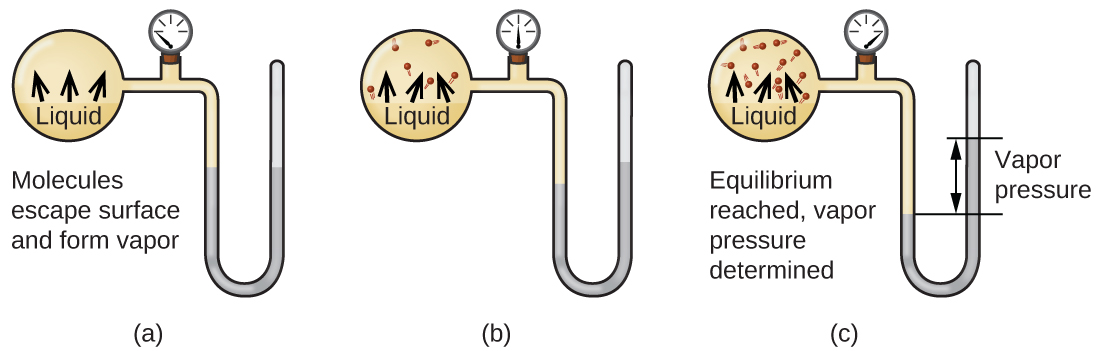
Now in an open container the liquid water or other liquid will slowly evaporate and the container will empty eventually. In which container can a dynamic equilibrium be established between water molecules in the liquid state and water molecules vapor state. If you imagine putting a pure liquid into a closed container the vapor pressure is initially close to zero and as a result the rate of condensation is also close to zero.
The water in the hermetic container partially evaporates until it reaches the saturation vapor pressure which is nothing other than the pressure at which the dynamic equilibrium between the liquid phase of water and the vapor phase is established. In thermodynamics and chemical engineering the vaporliquid equilibrium VLE describes the distribution of a chemical species between the vapor phase and a liquid phase. These vapors are recaptured by the liquid and the process is called condensation.
In which container will the water level remain constant. For this equilibrium to be established the system the closed flask containing both liquid and vapor must be at constant pressure. Figure 4 shows an equilibrium is pictured between liquid bromine Br 2 l the dark liquid and bromine vapor Br 2 g the orange gas.
Using their condensed structural. When the temperature of a contained liquid increases its vapor. To explain what a dynamic equilibrium is.
We know that the liquid molecules escape from the surface of the liquid and change into vapors. When a liquid is in dynamic equilibrium with its vapor phase the rates of evaporation and condensation are exactly equal to each other. To explain what equilibrium vapor pressure is and why it.
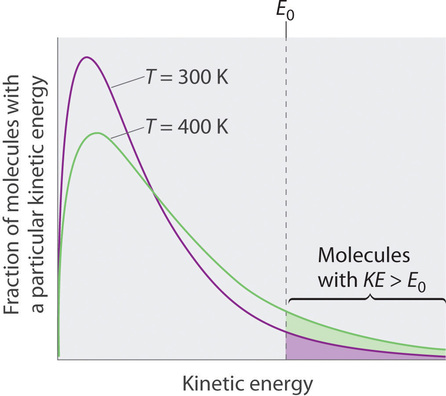
Athe vapor pressure of the liquid equals the external pressure B the liquid and vapor are at the same temperature Ca liquid vaporizes and condenses at the same rate D the liquid is entirely evaporated 2 The boiling points for a set of compounds in a homologous series can be qualitatively predicted using intermolecular force strengths. At normal temperatures the equilibrium between water and water vapour is established slowly. An equilibrium can be established for a physical changelike this liquid to gas transitionas well as for a chemical reaction.
Science Chemistry Chemistry by OpenStax 2015-05-04 What feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container. The concentration of a vapor in contact with its liquid especially at equilibrium is often expressed in terms of vapor pressure which will be a partial pressure a part of the total gas pressure if any other. D all the liquid has evaporated.
Why should this be so. From which container is it possible for all of the liquid water to disappear through evaporation. In a sealed bottle of soda carbon dioxide is present in both the liquidaqueous phase and the gaseous phase bubbles.
Youve witnessed an dynamic equilibrium example every time youve had a soda. So a dynamic equilibrium is reached when the rate of condensation rate off condensation is equal to the rate of evaporation. Condensation on the inside of the terrarium indicates that there is not a liquid-vapor equilibrium in the sealed terrarium.
Its called dynamic equilibrium because the liquid hasnt stopped changing into. It is not static because we actually have liquid that is vaporizing and vapor that is condensing back. Chemical reactions achieve dynamic equilibrium similar to phase equilibrium and characterized by an equilibrium constant Keq analogous to vapor pressure It occurs only in closed systems.
So weve got liquid that is evaporating into vapor. After a time in a closed partly filled container a liquid will evaporate and its vapor will condense at equal rates. Dynamic equilibrium doesnt just occur in chemistry labs though.
What feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container. This is an operation and vapor that is convincing into into liquid. Chapter 10 Problem 32E.
When equilibrium is established in the evaporation of a liquid into a sealed container we refer to it as dynamic equilibrium. That pressure in this case turned out to be 0031166 atm at 25. At constant temperature the rate of both the processes becomes equal.
Phase some molecules will re-enter the liquid phase and a situation will be established whereby the rate of evaporation will equal the rate of condensation ie a dynamic equilibrium between the liquid and gas phase will exist. A computer animation representing at the particulate level the dynamic equilibrium established between molecules in the liquid phase and molecules in the vapor gas phase of bromine. The established pressure in the gas phase is referred to as the equilibrium vapor pressure which is normally.
Dynamic equilibrium is when the rate of vapour and liquid being produced has stabilized and stopped changing. The molecules that have escaped are in the atmosphere over the liquid and like all gases exert a pressure. A dynamic equilibrium is established after.
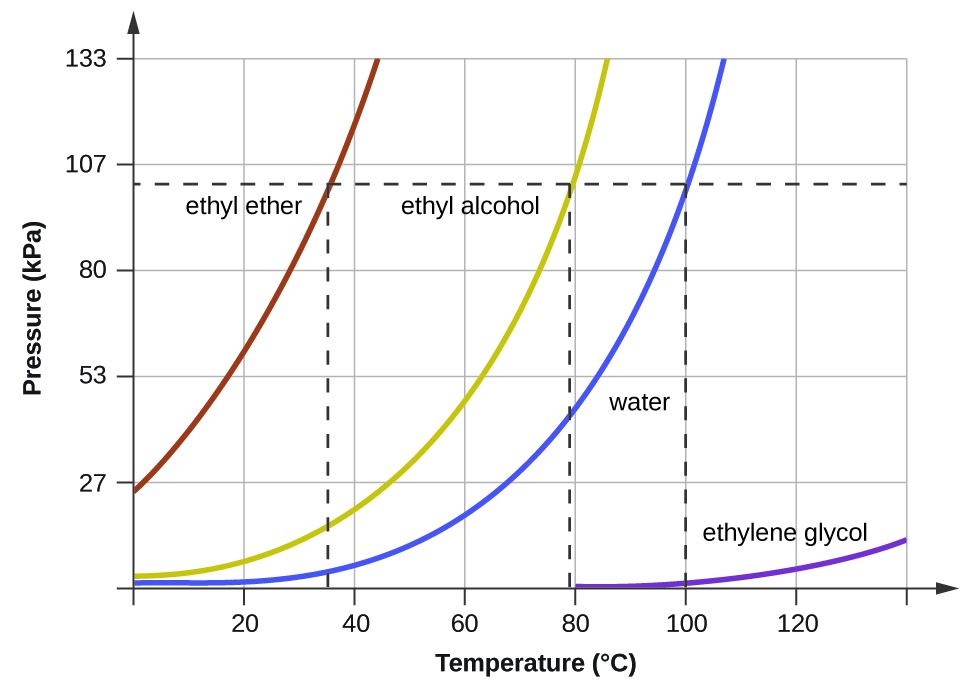
Equilibrium is established between a liquid and its vapor when a the liquid ceases to evaporate and the gas ceases to condense. Look at Figure 136b on page 391. Of course for water P SVP 1 atm AT 100 C.
Bromine Vapor Pressure Computer Animation. To explain why the system in which a liquid in a closed container comes to a dynamic equilibrium between the rate of evaporation and the rate of condensation. B cqual masses exist in the liquid and gas phases.
This is also not aesthetic. Because the container is sealed bromine vapor cannot escape and equilibrium is maintaineda sample of liquid bromine at. Dynamic equilibrium is a balance of opposing processes occurring at equal rates.
The partial pressure see Section 96 they exert is known as the vapor pressure of the liquid. C cqual concentrations in molarity exist in the liquid and gas phases.

Entropy Balance For Open Systems Entropy Thermodynamics Potential Energy
10 3 Phase Transitions Chemistry

Equilibrium Vapor Pressure An Overview Sciencedirect Topics
3 6 1 Vapor Pressure Chemistry Libretexts

Lesson Explainer Vapor Pressure Nagwa

Vapor Liquid Equilibrium During Distillation Epic Modular Process

Equilibrium Vapor Pressure An Overview Sciencedirect Topics

Air Bubble Rising In Water Computer Aided Engineering Ansys Bubbles

Lesson Video Vapor Pressure Nagwa

Salts Solubility Solubility Salt Activity Board
11 5 Vaporization And Vapor Pressure Chemistry Libretexts

Equilibrium Vapor Pressure An Overview Sciencedirect Topics

Unit 11 Thermodynamics Chapter 16 Thermodynamics Definition Definition A Study Of Heat Transfer That Accompanies Ch Thermodynamics Chemistry Electron Affinity

Common Ion Effect Solubility Science Chemistry Easy Science

Throttle Flow Simulation With Openfoam Free Download Here Http Fetchcfd Com View Project 335 Computer Aided Engineering Computer Simulation Simulation



Comments
Post a Comment